The Hofmann Elimination. Amines can participate in E2 reactions to form alkenes. However, just like the OH group of alcohols, the NH 2, or any other amino group, is a quite strong base and needs to be first converted into a good leaving group. This is achieved by methylation of the nitrogen with excess of methyl iodide.
Amines and Heterocycles – ppt video online download
Kumada Coupling Reaction 13m. Negishi Coupling Reaction 16m. Buchwald-Hartwig Amination Reaction 19m. Eglinton Reaction 17m. For each reaction, decide whether substitution or elimination (or both) is possible, and predict the products you expect. Label the major products. a. 1−bromo−1−methylcyclohexane + NaOH in acetone.

Source Image: youtube.com
Download Image
Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. It states that in an elimination reaction the major product is the more stable alkene with the more highly substituted double bond. This situation is illustrated by the 2-bromobutane and 2-bromo-2,3-dimethylbutane elimination examples given below.
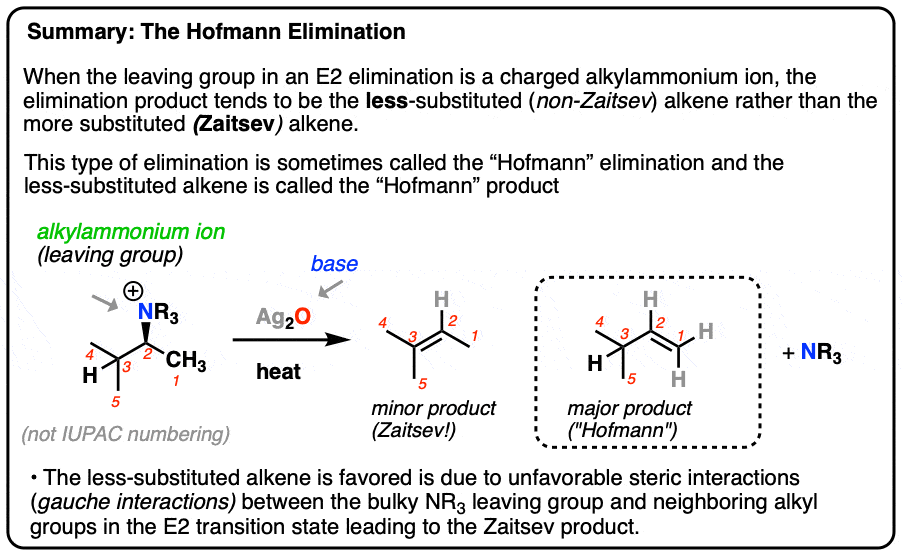
Source Image: masterorganicchemistry.com
Download Image
76 Named Reactions ideas | organic chemistry study, organic chemistry, chemistry notes [1] [2] The reaction starts with the formation of a quaternary ammonium iodide salt by treatment of the amine with excess methyl iodide ( exhaustive methylation ), followed by treatment with silver oxide and water to form a quaternary ammonium hydroxide.
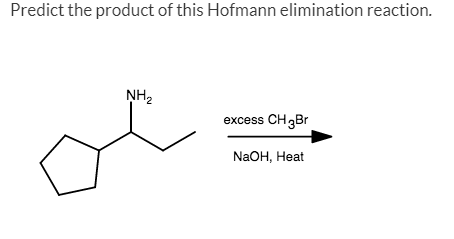
Source Image: chegg.com
Download Image
Predict The Product Of This Hofmann Elimination Reaction
[1] [2] The reaction starts with the formation of a quaternary ammonium iodide salt by treatment of the amine with excess methyl iodide ( exhaustive methylation ), followed by treatment with silver oxide and water to form a quaternary ammonium hydroxide. This video provides a test question on E2 elimination reactions with a focus on distinguishing the zaitsev product and the hoffman product.Access The Full Vi
Solved Predict the product of this Hofmann elimination | Chegg.com
Unlike what happens in other E2 reactions, the major product of Hofmann elimination is the less highly substituted alkene rather than the more highly substituted one, as shown by the reaction of (1-methylbutyl)trimethylammonium hydroxide to give 1-pentene rather than the alternative 2-pentene. Hofmann elimination ~ Name-Reaction.com

Source Image: name-reaction.com
Download Image
SN E Flowchart PDF | PDF | Chemical Substances | Organic Chemistry Unlike what happens in other E2 reactions, the major product of Hofmann elimination is the less highly substituted alkene rather than the more highly substituted one, as shown by the reaction of (1-methylbutyl)trimethylammonium hydroxide to give 1-pentene rather than the alternative 2-pentene.

Source Image: scribd.com
Download Image
Amines and Heterocycles – ppt video online download The Hofmann Elimination. Amines can participate in E2 reactions to form alkenes. However, just like the OH group of alcohols, the NH 2, or any other amino group, is a quite strong base and needs to be first converted into a good leaving group. This is achieved by methylation of the nitrogen with excess of methyl iodide.

Source Image: slideplayer.com
Download Image
76 Named Reactions ideas | organic chemistry study, organic chemistry, chemistry notes Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. It states that in an elimination reaction the major product is the more stable alkene with the more highly substituted double bond. This situation is illustrated by the 2-bromobutane and 2-bromo-2,3-dimethylbutane elimination examples given below.

Source Image: pinterest.com
Download Image
Hofmann’s Elimination Reaction. – YouTube 23: Amines

Source Image: youtube.com
Download Image
Zaitsev’s Rule Definition, Characteristics & Product – Lesson | Study.com [1] [2] The reaction starts with the formation of a quaternary ammonium iodide salt by treatment of the amine with excess methyl iodide ( exhaustive methylation ), followed by treatment with silver oxide and water to form a quaternary ammonium hydroxide.

Source Image: study.com
Download Image
The Hofmann Elimination. a quaternary ammonium hydroxide is the reactant and an alkene is the product is an anti elimination the leaving group is a trialkylamine. – ppt download This video provides a test question on E2 elimination reactions with a focus on distinguishing the zaitsev product and the hoffman product.Access The Full Vi

Source Image: slideplayer.com
Download Image
SN E Flowchart PDF | PDF | Chemical Substances | Organic Chemistry
The Hofmann Elimination. a quaternary ammonium hydroxide is the reactant and an alkene is the product is an anti elimination the leaving group is a trialkylamine. – ppt download Kumada Coupling Reaction 13m. Negishi Coupling Reaction 16m. Buchwald-Hartwig Amination Reaction 19m. Eglinton Reaction 17m. For each reaction, decide whether substitution or elimination (or both) is possible, and predict the products you expect. Label the major products. a. 1−bromo−1−methylcyclohexane + NaOH in acetone.
76 Named Reactions ideas | organic chemistry study, organic chemistry, chemistry notes Zaitsev’s Rule Definition, Characteristics & Product – Lesson | Study.com 23: Amines