What is the pH of 0.1 M ammonia? Solution Verified Answered 8 months ago Create an account to view solutions Recommended textbook solutions Chemistry: The Molecular Nature of Matter and Change 7th Edition • ISBN: 9780073511177 (2 more) Patricia Amateis, Silberberg 6,032 solutions Chemistry: The Central Science
FTUDI 1.2 Calculate the pH of a 0.10M ammonia solution. Calculate the pH after 50.0 mL of this solution is treated with 25.0 mL of 0.10M HCl. The dissociation constant of ammonia,
Std molar entropy (S … = 0.0042 M, [NH 3] = 0.9958 M, and pH = 14 + log 10 [OH … Diluted (1-3%) ammonia is also an ingredient of numerous cleaning agents, including many window cleaning formulas. Because aqueous ammonia is a gas dissolved in water, as the water evaporates from a window, the gas evaporates also, leaving the window streak
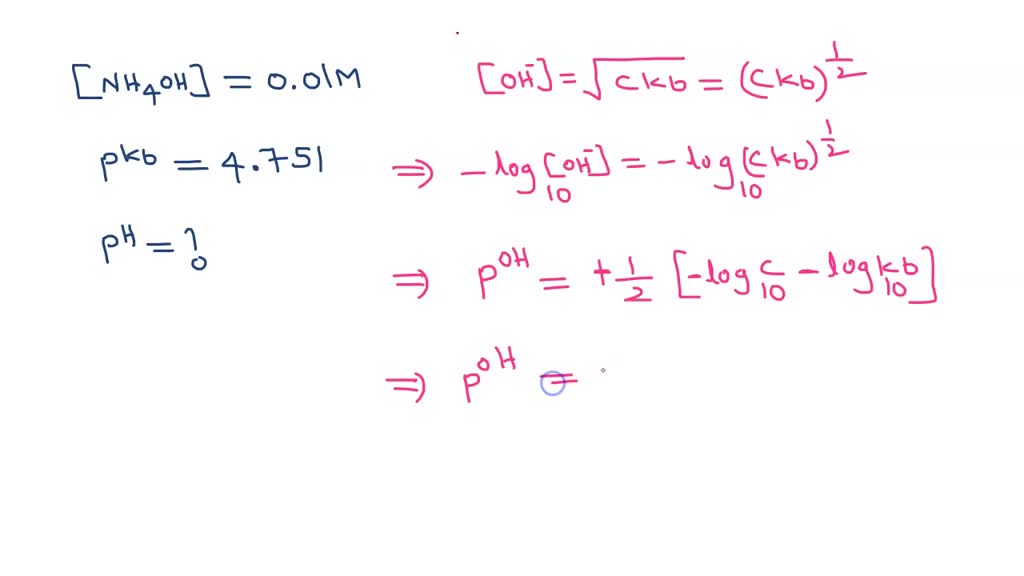
Source Image: numerade.com
Download Image
0.1 N: 11.1: Ammonia: 0.01 N: 10.6: Barbital sodium: 0.1N: 9.4: Borax: 0.01 N: 9.2: Calcium carbonate: saturated: 9.4: … pH range vs. color change for acid and base indicators – together with pKa and structures of the indicators. … pKa (the negative logarithm of the acid dissociation constant), molecular structures, molar weights, density

Source Image: toppr.com
Download Image
SOLVED: What is the pH of 0.1M ammonium hydroxide? What is the pH of 0.001 M sodium hydroxide? Are they similar? Would you expect the pH of 0.1M ammonium hydroxide to be An ammonia solution is 9. 9 % ammonia by mass and has a density of 0. 9 9 g/mL. Calculate the pH of the solution. Calculate the pH of the solution. K b ( N H 4 O H ) = 1 . 7 × 1 0 − 5 .

Source Image: pubs.acs.org
Download Image
What Is The Ph Of 0.1 Molar Ammonia
An ammonia solution is 9. 9 % ammonia by mass and has a density of 0. 9 9 g/mL. Calculate the pH of the solution. Calculate the pH of the solution. K b ( N H 4 O H ) = 1 . 7 × 1 0 − 5 . So 0.050 moles divided by 0.150 liters gives the concentration of acetic acid a 0.33 molar. For the acetate anion, we also have 0.050 moles, and the total volume of solution is also 0.150 liters. Therefore, the concentration of the acetate anion is also 0.33 molar. To find the pH of the buffer solution, we can use the Henderson-Hasselbalch
Growth of Nitrosococcus-Related Ammonia Oxidizing Bacteria Coincides with Extremely Low pH Values in Wastewater with High Ammonia Content | Environmental Science & Technology
1 Answer Ernest Z. Feb 6, 2017 The pH = 11.3. Explanation: To calculate the new concentration of NaOH in the ammonia solution, you can use the dilution formula ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a c1V 1 = c2V 2 a a ∣∣ −−−−−−−−−−−−−−− We can rearrange this formula to get c2 = c1 × V 1 V 2 c1 = 0.1 mol/L;V 1 = 0.1 mL e No. 101 PCB-20180301 84. An aqueous mixture room temperature is 0.1 M with respect to NH OH. PKof aqueous ammonia as a base is 5: the pH of the mixture is : (

Source Image: toppr.com
Download Image
Calculate the ph of 0.1m ammonia solution calculate the ph after 50ml of this solution is treated with 25 ml – Brainly.in 1 Answer Ernest Z. Feb 6, 2017 The pH = 11.3. Explanation: To calculate the new concentration of NaOH in the ammonia solution, you can use the dilution formula ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a c1V 1 = c2V 2 a a ∣∣ −−−−−−−−−−−−−−− We can rearrange this formula to get c2 = c1 × V 1 V 2 c1 = 0.1 mol/L;V 1 = 0.1 mL

Source Image: brainly.in
Download Image
FTUDI 1.2 Calculate the pH of a 0.10M ammonia solution. Calculate the pH after 50.0 mL of this solution is treated with 25.0 mL of 0.10M HCl. The dissociation constant of ammonia, What is the pH of 0.1 M ammonia? Solution Verified Answered 8 months ago Create an account to view solutions Recommended textbook solutions Chemistry: The Molecular Nature of Matter and Change 7th Edition • ISBN: 9780073511177 (2 more) Patricia Amateis, Silberberg 6,032 solutions Chemistry: The Central Science

Source Image: toppr.com
Download Image
SOLVED: What is the pH of 0.1M ammonium hydroxide? What is the pH of 0.001 M sodium hydroxide? Are they similar? Would you expect the pH of 0.1M ammonium hydroxide to be 0.1 N: 11.1: Ammonia: 0.01 N: 10.6: Barbital sodium: 0.1N: 9.4: Borax: 0.01 N: 9.2: Calcium carbonate: saturated: 9.4: … pH range vs. color change for acid and base indicators – together with pKa and structures of the indicators. … pKa (the negative logarithm of the acid dissociation constant), molecular structures, molar weights, density
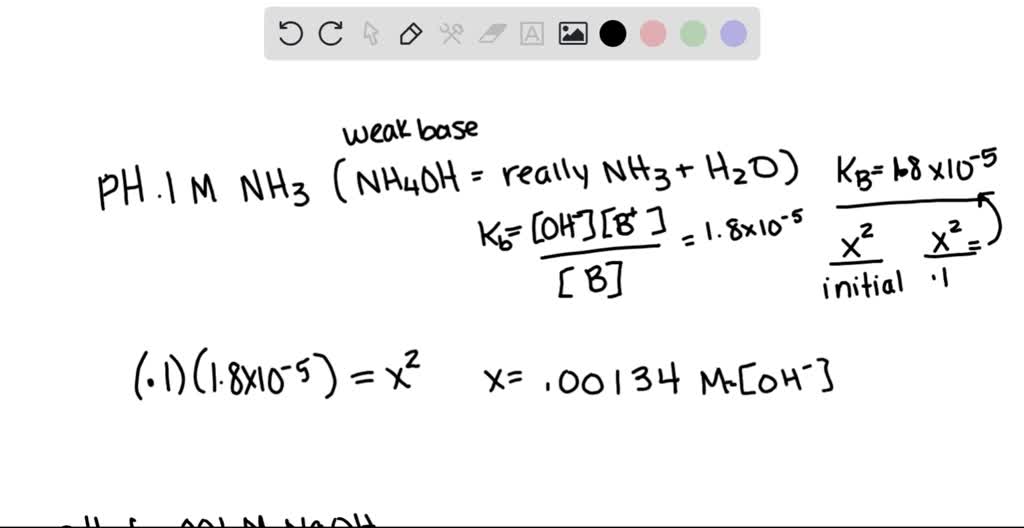
Source Image: numerade.com
Download Image
TOGWT: Detailing Chemicals Identification So we’re gonna lose 0.06 molar of ammonia, ’cause this is reacting with H 3 O plus. And so after neutralization, we’re left with 0.18 molar for the concentration of ammonia. And whatever we lose for ammonia, we gain for ammonium since ammonia turns into ammonium. So we’re going to gain 0.06 molar for our concentration of ammonium after

Source Image: togwt1980.blogspot.com
Download Image
What is the pH of a 0.1 M ammonia solution? An ammonia solution is 9. 9 % ammonia by mass and has a density of 0. 9 9 g/mL. Calculate the pH of the solution. Calculate the pH of the solution. K b ( N H 4 O H ) = 1 . 7 × 1 0 − 5 .
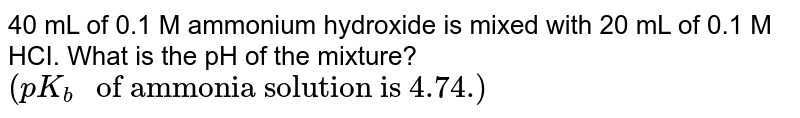
Source Image: doubtnut.com
Download Image
Quick video: Calculating the pH of a 0.1M NH3 ammonia solution – YouTube So 0.050 moles divided by 0.150 liters gives the concentration of acetic acid a 0.33 molar. For the acetate anion, we also have 0.050 moles, and the total volume of solution is also 0.150 liters. Therefore, the concentration of the acetate anion is also 0.33 molar. To find the pH of the buffer solution, we can use the Henderson-Hasselbalch

Source Image: m.youtube.com
Download Image
Calculate the ph of 0.1m ammonia solution calculate the ph after 50ml of this solution is treated with 25 ml – Brainly.in
Quick video: Calculating the pH of a 0.1M NH3 ammonia solution – YouTube Std molar entropy (S … = 0.0042 M, [NH 3] = 0.9958 M, and pH = 14 + log 10 [OH … Diluted (1-3%) ammonia is also an ingredient of numerous cleaning agents, including many window cleaning formulas. Because aqueous ammonia is a gas dissolved in water, as the water evaporates from a window, the gas evaporates also, leaving the window streak
SOLVED: What is the pH of 0.1M ammonium hydroxide? What is the pH of 0.001 M sodium hydroxide? Are they similar? Would you expect the pH of 0.1M ammonium hydroxide to be What is the pH of a 0.1 M ammonia solution? So we’re gonna lose 0.06 molar of ammonia, ’cause this is reacting with H 3 O plus. And so after neutralization, we’re left with 0.18 molar for the concentration of ammonia. And whatever we lose for ammonia, we gain for ammonium since ammonia turns into ammonium. So we’re going to gain 0.06 molar for our concentration of ammonium after